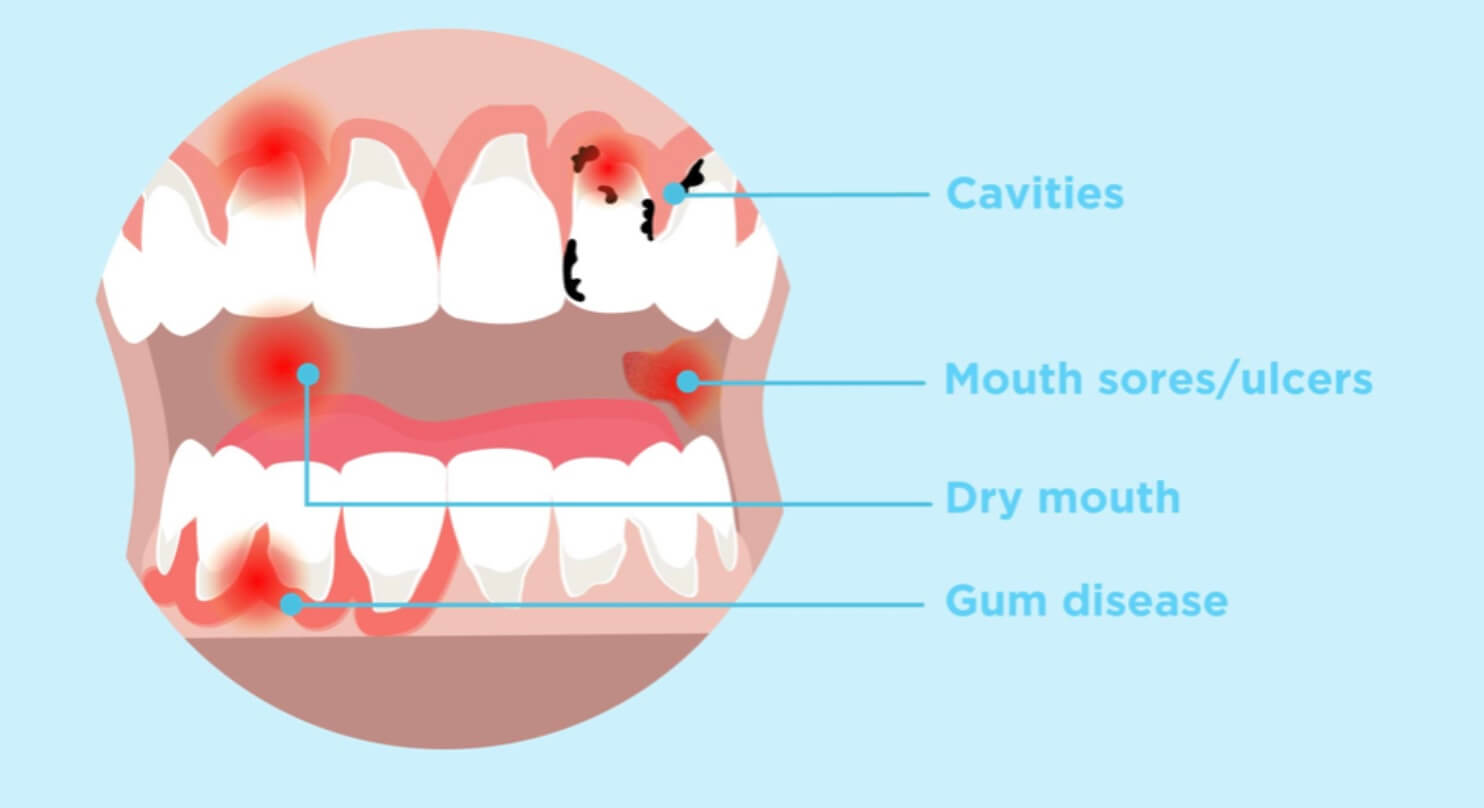
The FDA issued a MedWatch Alert warning clinicians of patients undergoing treatment with dissolvable buprenorphine products to treat opioid use disorder (OUD) and/or pain about the risk for dental problems associated with the medications.
“Dental problems (including tooth decay, cavities, dental abscesses/infection, tooth erosion and, in some cases, total tooth loss), have been reported even in patients with no history of dental issues,” according to the FDA in the alert.
Buprenorphine is approved as an under-the-tongue combination buprenorphine-naloxone oral tablet to treat OUD (Suboxone, Indivior) and as a film to be placed inside the cheek to treat pain (Belbuca, Biodelivery Sciences).
The FDA stated that while there are no risk predictors for patients taking dissolvable buprenorphine with regard to related dental problems, “the benefits of buprenorphine medicines for OUD and pain clearly outweigh the risks, and are important tools in treating these conditions,” according to the agency.
It’s advised that patients using buprenorphine formulations that dissolve in the mouth to take precautionary measures, such as rinsing with water after buprenorphne use; avoiding brushing their teeth for an hour after taking a dose to reduce the risk for tooth damage; and undergoing regular dental checkups.